Abstract
Breakthrough progress in CAR-T treatment for B cell Acute lymphoblastic leukemia (B-ALL) has led to major improvements in B-ALL patient survival. However, recent clinical studies involving B-ALL patients have shown that CAR-T related adverse events have a negative impact on patient prognosis. Multiple CAR-T related cytotoxic effects such as cytokine release syndrome (CRS) and neutropenia were especially shown to co-occur and were detrimental to the survival and recovery of B-ALL patients. This has consequently fueled interest in developing novel in vivo models to emulate these conditions. Currently, there are no models that can recapitulate multiple CAR-T related cytotoxic effects in a single in vivo model.
To establish a mouse model that can be used to study both CRS and Neutropenia, we explored the role of IL-2Rα in inducing CAR-T related toxicities. We hypothesized that knockout of IL2Rα will lead to severe CRS after CAR-T infusion, owing to its crucial role in the regulation of cytokine-mediated inflammation, and its abundant expression in regulatory T cells that control self -tolerance and regulate T cell expansion. To prove our hypothesis, a pre-established IL-2Rα -/- or IL-2Rα KO mouse model was used, in which exons 2 and 3 of IL-2Rα gene that encode the binding site for IL-2, is replaced by a neomycin resistance gene driven by the PKG promoter. We injected 1 million murine B-ALL cells (Eμ-ALL) a week before inoculating 1 million CD19-28z CAR-T cells in IL2Rα KO and Wild type (WT) mice, and profiled for serum cytokine levels of IL6, IFNg and TNFα, followed by complete blood profiling (CBC) for weeks 1-4. Furthermore, we repeated this study without injecting Eμ-ALL cells and with CAR-T cells only (2 million), to understand whether CAR-T alone can impact CRS and Neutropenia.
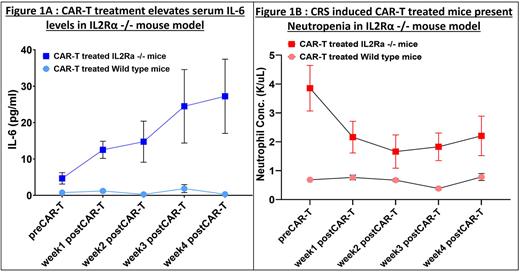
We found that KO mice injected with only CAR-T cells, developed severe CRS based on their elevated serum levels of IL6 (paired t test, p=0.006) (Fig 1A), IFNg (paired t test, p=0.033) and TNFa (paired t test, p=0.047) in week 4 post CAR-T vs CAR-T untreated mice. The difference in cytokine levels in WT mice under similar conditions was not statistically significant. Histopathological analysis confirmed that the IL2Rα KO mice developed secondary hemophagocytic lymphohistiocytosis, or macrophage activation (sHLH/MAS) that manifest during CAR-T associated CRS. To validate if the characteristics observed in IL2Rα KO mice are a consequence of CRS, we treated IL2Rα KO mice with 0.3 mg anti-mouse IL6-R mAb per mouse (mouse equivalent of Tocilizumab used in CRS management), 5 hours pre-CAR-T and biweekly throughout the study. We observed a significant improvement in the survival of CAR-T infused IL2Rα KO mice that were treated with anti-mouse IL6-R mAb compared to the untreated group (Gehan-Breslow-Wilcoxon test, p=0.031), thus confirming the incidence of severe CRS in these mice.
Complete blood profiling of CAR-T alone treated IL2Rα KO mice showed a significant decrease in their Neutrophil concentration (paired t test, p=0.004), which did not occur in WT mice (Fig1B). This indicated that loss of IL2Rα not only regulates CRS but is also crucial for Neutrophils. We further validated the onset of Neutropenia in the KO mice by assessing whether CAR-T affects the turnover rate (rate of proliferation, maturation and apoptosis) of Neutrophils. Neutrophils isolated from bone marrow of KOs and WTs pre-CAR-T and 1-4 weeks post CAR-T treatment, showed a decrease in BrDU+ neutrophils in CAR-T treated KO mice only (paired t-test, p<0.0001), indicative of a decrease in % Neutrophil proliferation. Similarly, CAR-T treated KO mice showed increase in Annexin V+ neutrophils (paired t test,p=0.034) that is indicative of an increase in % Neutrophil apoptosis. Furthermore, CAR-T treatment impaired the maturation status of Neutrophils (measured by the % of Ly6G+CXCR2+Neutrophils), confirming the prevalence of Neutropenia alongside CRS (paired t-test, p=0.0005)
We developed a novel IL2Rα KO model that recapitulates co-occurrence of CAR-T induced CRS and neutropenia observed in B-ALL patients. Our model will help understand the crosstalk between CAR-T and host immune components such as macrophages and Neutrophils that are altered during CRS and Neutropenia. Ongoing experiments are directed towards identifying key mediators of both CRS and Neutropenia during CAR-T therapy, which will improve treatment intervention during CAR-T associated cytotoxicity.
Disclosures
Yongliang:Iovance Therapeutics: Current Employment, Current equity holder in publicly-traded company; BeiGene: Current equity holder in publicly-traded company; Gamida Cell: Current equity holder in publicly-traded company; Alaunos Therapeutics: Current equity holder in publicly-traded company; SQZ Biotechnologies: Current equity holder in publicly-traded company. Jain:MyeloidTx: Consultancy; BMS: Consultancy; Novartis: Consultancy; Incyte: Research Funding; Kite Pharma: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.